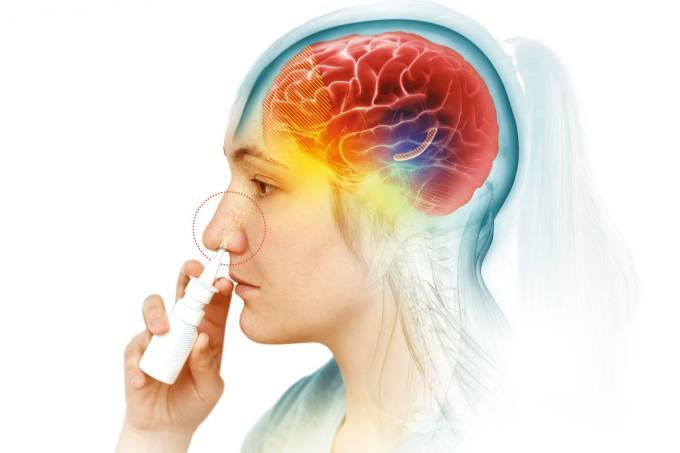
Anvisa approves first nasal spray for depression
Inhalable spray is the biggest medical novelty since the first antidepressants in the 1950s
Anvisa (National Health Surveillance Agency) approved this Tuesday, the 3rd, the first inhalable spray against depression. Escetamine hydrochloride is indicated for the treatment of adults with resistant depression, that is, people who have not responded to two previous therapies, or who have suicidal behavior or ideation.
Spravato, the product's commercial name, is considered the greatest advance in science against depression in half a century. “This is the first treatment with a truly innovative mechanism of action approved in decades and offers a new option to respond to the unmet needs of patients and the medical community”, explains psychiatrist Pedro do Prado Lima , from the Instituto do C brain of the Pontifical Catholic University of Rio Grande do Sul.
Developed by Janssen, a pharmacist at Johnson & Johnson, the nasal spray has faster brain action than any other medication on the market, which is a key factor for patients with intentions suicidal. The drug starts to take effect in a few hours. Common antidepressants show their first results after about a month of treatment.
The drug works differently than currently available therapies for the disease. The existing antidepressants currently act on monoamines, such as serotonin, dopamine and norepinephrine. Intranasal escetamine acts on N-methyl-D-aspartate (NMDA) glutamate receptors, which help restore synaptic connections in brain cells of people with depression. The indication is that it is used in conjunction with an oral antidepressant.
The results of two phase 3 clinical studies showed that escetamine in conjunction with standard therapy reduced depressive symptoms within 24 hours after the first dose. After one month of treatment, 70% of patients showed improvement in the most severe symptoms. However, the product was not able to prevent suicide or reduce suicidal ideation or behavior. Thus, the use of the drug does not dispense with the need for hospitalization in patients with clinical indication.
The main side effects seen during treatment with escetamine include dissociation, dizziness, nausea, sedation, spinning sensation, blurred vision, reduced sense of touch and sensation, anxiety, lack of energy , high blood pressure, vomiting, paraesthesia and feeling drunk.
“We are very proud to make intranasal ketamine available to Brazilian patients with very disabling types of depression, for whom treatment options were scarce,” says psychiatrist Fabio Lawson, medical director of Janssen Brasil.
To ensure its correct use, intranasal ketamine will be administered in authorized hospitals and clinics, always under the supervision of a health professional. The price is not yet defined in Brazil.
Source: Material removed from the site See
Photo: Art | See